Estudios clínicos
La mediana de supervivencia global (SG) más larga que se ha registrado en el cáncer de mama metastásico (CMM) HR+/HER2- en 3 de 3 estudios clínicos. 1,2,3
En las siguientes gráficas se muestra como KISQALI® tiene un beneficio consistente y significativo en la supervivencia global en los tres estudios de fase III. 1,2,3,4,5,6
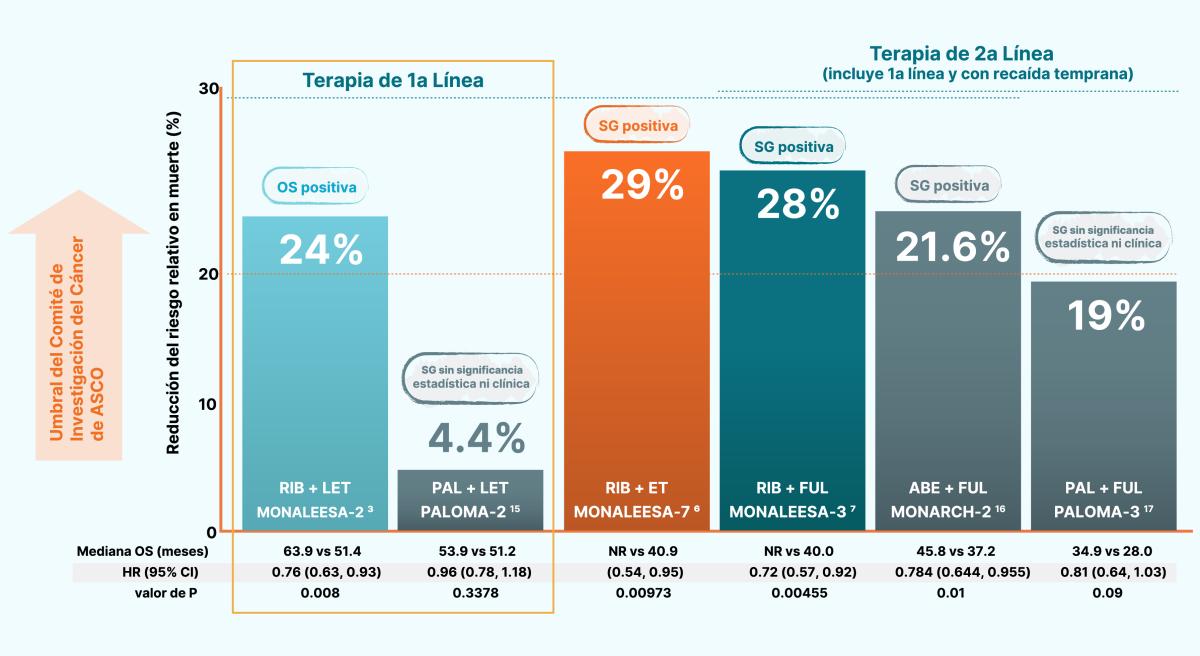

KISQALI® + Fulvestrant en pacientes posmenopáusicas sin tratamiento previo o con solo 1L de TE. 2

KISQALI® + Goserelina + IA (letrozol o anastrozol) o tamoxifeno en mujeres pre o perimenopáusicas. 3
Referencias
- Hortobagyi GN, Stemmer SM, Burris HA, Yap Y-S, Sonke GS, Hart L, et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med [Internet]. 2022;386(10):942–50. Available from: http://dx.doi.org/10.1056/nejmoa2114663
- Slamon DJ, Neven P, Chia S, Jerusalem G, De Laurentiis M, Im S, et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol [Internet]. 2021;32(8):1015–24. Available from: http://dx.doi.org/10.1016/j.annonc.2021.05.353
- Im S-A, Lu Y-S, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med [Internet]. 2019;381(4):307–16. Available from: http://dx.doi.org/10.1056/nejmoa1903765.
- Finn, R. S. et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): Analyses from PALOMA-2. J Clin Oncol 40, LBA1003 (2022).
- Llombart-Cussac, A. et al. Abstract PD13-11: PD13-11 Final Overall Survival Analysis of Monarch 2 : A Phase 3 trial of Abemaciclib Plus Fulvestrant in Patients with Hormone Receptor-Positive, HER2-Negative Advanced Breast Cancer. Cancer Res 83, PD13-11 (2023).
- Turner, N. C. et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N Engl J Med 379, 1926-1936 (2018).
- Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol [Internet]. 2018;29(7):1541–7.
- O’Shaughnessy J, Beck T, Chia S, Isaacs C, DeLaurentiis M, Kummel S,et. al. Efficacy and safety of first-line ribociclib + letrozole in patients with de novo metastatic disease and late recurrence from (neo)adjuvant therapy in MONALEESA-2 [Internet]. 2023. European Society for Medical Oncology Breast Cancer Congress. Available from: https://www.medicalcongress.novartisoncology.com/ESMOBC23/BC/pdf/OShaughnessy_196P_Poster.pdf#toolbar=0
- Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im S-A, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: MONALEESA-3. J Clin Oncol [Internet]. 2018;36(24):2465–72. Available from: http://dx.doi.org/10.1200/jco.2018.78.9909
- Tripathy D, Im S-A, Colleoni M, Franke F, Bardia A, Harbeck N, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol [Internet]. 2018;19(7):904–15. Available from: http://dx.doi.org/10.1016/s1470-2045(18)30292-4
- Lu Y-S, Im S-A, Colleoni M, Franke F, Bardia A, Cardoso F, et al. Updated overall survival of ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with HR+/HER2− advanced breast cancer in MONALEESA-7: A phase III randomized clinical trial. Clin Cancer Res [Internet]. 2022;28(5):851–9. Disponible en: http://dx.doi.org/10.1158/1078-0432.ccr-21-3032
Material exclusivo para profesional de la salud. P3 MX2312061396 No. Aviso COFEPRIS 2309052002C00231 Fecha de vigencia: 26/12/2025
*Las imágenes no corresponden a pacientes reales.


